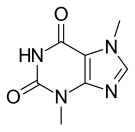
Theobromine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | xantheose diurobromine 3,7-dimethylxanthine 3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione |
| Dependence liability | None |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic demethylation and oxidation |
| Elimination half-life | 6–8 hours[1][2] |
| Excretion | Renal (10% unchanged, rest as metabolites) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.359 |
| Chemical and physical data | |
| Formula | C7H8N4O2 |
| Molar mass | 180.167 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
| Identifiers | |
|---|---|
| ECHA InfoCard | 100.001.359 |
CompTox Dashboard (EPA)
|
|
| Properties | |
| Appearance | white solid |
| Density | 1.524 g/cm3[3] |
| Melting point | 351 °C (664 °F; 624 K) |
| 330 mg/L) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Theobromine, also known as xantheose, is the principal alkaloid of Theobroma cacao (cacao plant).[4] Theobromine is slightly water-soluble (330 mg/L) with a bitter taste.[5] In industry, theobromine is used as an additive and precursor to some cosmetics.[4] It is found in chocolate, as well as in a number of other foods, including tea (Camellia sinensis), some American hollies (yaupon and guayusa) and the kola nut. It is a white or colourless solid, but commercial samples can appear yellowish.[5]
Structure
[edit]Theobromine is a flat molecule,[3] a derivative of purine and an isomer of theophylline.[6] It is also classified as a dimethyl xanthine.[5][7] Related compounds include theophylline, caffeine, paraxanthine, and 7-methylxanthine, each of which differ in the number or placement of the methyl groups.[5]
History
[edit]Theobromine was first discovered in 1841[8] in cacao beans by the chemist A. Woskresensky.[9] Synthesis of theobromine from xanthine was first reported in 1882 by Hermann Emil Fischer.[10][11][12]
Etymology
[edit]Theobromine is derived from Theobroma, the name of the genus of the cacao tree, with the suffix -ine given to alkaloids and other basic nitrogen-containing compounds.[13] That name in turn is made up of the Greek roots theo ("god") and broma ("food"), meaning "food of the gods".[14]
Despite its name, the compound contains no bromine, which is based on Greek bromos ("stench").
Sources
[edit]
Theobromine is the primary alkaloid found in cocoa and chocolate. Cocoa butter only contains trace amounts of theobromine. There are usually higher concentrations in dark than in milk chocolate.[15]
There are approximately 60 milligrams (1 grain) of theobromine in 28 grams (1 oz) of milk chocolate,[16] while the same amount of dark chocolate contains about 200 milligrams (3 grains).[17] Cocoa beans naturally contain approximately 1% theobromine.[18]
Plant species and components with substantial amounts of theobromine are:[19][20]
- Theobroma cacao – seed and seed coat
- Theobroma bicolor – seed coat
- Ilex paraguariensis – leaf
- Ilex guayusa – leaf
- Ilex vomitoria – leaf
- Camellia sinensis – leaf
Theobromine can also be found in trace amounts in the kola nut, the guarana berry, yerba mate (Ilex paraguariensis),[21] and the tea plant.[22]
The mean theobromine concentrations in cocoa and carob products are:[23][24]
| Item | Mean theobromine per 100 g |
|---|---|
| Cocoa powder | 2060 mg |
| Cocoa beverages | 266 mg |
| Chocolate toppings | 195 mg |
| Chocolate bakery products | 147 mg |
| Cocoa cereals | 69.5 mg |
| Chocolate ice creams | 62.1 mg |
| Chocolate milks | 22.6 mg |
| Carob products | 0.00–50.4 mg |
Biosynthesis
[edit]Theobromine is a purine alkaloid derived from xanthosine, a nucleoside. Cleavage of the ribose and N-methylation yields 7-methylxanthosine. 7-Methylxanthosine in turn is the precursor to theobromine, which in turn is the precursor to caffeine.[25]
Pharmacology
[edit]
Even without dietary intake, theobromine may occur in the body as it is a product of the human metabolism of caffeine, which is metabolised in the liver into 12% theobromine, 4% theophylline, and 84% paraxanthine.[26]
In the liver, theobromine is metabolized into xanthine and subsequently into methyluric acid.[27] Important enzymes include CYP1A2 and CYP2E1.[28] The elimination half life of theobromine is between 6 and 8 hours.[1][2]
Unlike caffeine, which is highly water-soluble, theobromine is only slightly water-soluble and is more fat soluble, and thus peaks more slowly in the blood. While caffeine peaks after only 30 minutes, theobromine requires 2–3 hours to peak.[29]
The primary mechanism of action for theobromine inside the body is inhibition of adenosine receptors.[5] Its effect as a phosphodiesterase inhibitor[30] is thought to be small.[5]
Effects
[edit]Humans
[edit]Theobromine has no significant stimulant effect on the human central nervous system.[4] It is a bronchodilator and causes relaxation of vascular smooth muscle.[4] It is not currently used as a prescription drug.[5] The amount of theobromine found in chocolate is small enough that chocolate can, in general, be safely consumed by humans.
Compared with caffeine, theobromine is weaker in both its inhibition of cyclic nucleotide phosphodiesterases and its antagonism of adenosine receptors.[4][31] The potential phosphodiesterase inhibitory effect of theobromine is seen only at amounts much higher than what people normally would consume in a typical diet including chocolate.[32]
Toxicity
[edit]At doses of 0.8–1.5 g/day (50–100 g cocoa), sweating, trembling and severe headaches were noted, with limited mood effects found at 250 mg/day.[33]
Also, chocolate may be a factor for heartburn in some people because theobromine may affect the esophageal sphincter muscle in a way that permits stomach acids to enter the esophagus.[34]
Animals
[edit]Theobromine is the reason chocolate is poisonous to dogs. Dogs and other animals that metabolize theobromine (found in chocolate) more slowly[35] can succumb to theobromine poisoning from as little as 50 g (1.8 oz) of milk chocolate for a smaller dog and 400 g (14 oz), or around nine 44-gram (1.55 oz) small milk chocolate bars, for an average-sized dog. The concentration of theobromine in dark chocolates (about 10 g/kg (0.16 oz/lb)) is up to 10 times that of milk chocolate (1 to 5 g/kg (0.016 to 0.080 oz/lb)), meaning dark chocolate is far more toxic to dogs per unit weight or volume than milk chocolate.[citation needed]
The median lethal dose of theobromine for dogs is 100–200 mg/kg (0.0016–0.0032 oz/lb); therefore, a 10 kg (22 lb) dog would need to consume a minimum of 200 g (7.1 oz) of the most theobromine-rich (5 g/kg (0.080 oz/lb)) dark chocolate, or a maximum of 1 kg (2.2 lb) (of theobromine-rich milk chocolate), to have a 50% chance of receiving a lethal dose. However, even 40 g (1.4 oz) of milk chocolate may induce vomiting and diarrhea.[36]
The same risk is reported for cats as well,[37] although cats are less likely to ingest sweet food, as cats lack sweet taste receptors.[38] Complications include digestive issues, dehydration, excitability, and a slow heart rate. Later stages of theobromine poisoning include epileptic-like seizures and death. If caught early on, theobromine poisoning is treatable.[39] Although not common, the effects of theobromine poisoning can be fatal.[38]
See also
[edit]References
[edit]- ^ a b Drouillard DD, Vesell ES, Dvorchik BH (March 1978). "Studies on theobromine disposition in normal subjects. Alterations induced by dietary abstention from or exposure to methylxanthines". Clinical Pharmacology and Therapeutics. 23 (3): 296–302. doi:10.1002/cpt1978233296. PMID 627135. S2CID 10519385.
- ^ a b Lelo A, Birkett DJ, Robson RA, Miners JO (August 1986). "Comparative pharmacokinetics of caffeine and its primary demethylated metabolites paraxanthine, theobromine and theophylline in man". British Journal of Clinical Pharmacology. 22 (2): 177–182. doi:10.1111/j.1365-2125.1986.tb05246.x. PMC 1401099. PMID 3756065.
- ^ a b Ford KA, Ebisuzaki Y, Boyle PD (1998). "Methylxanthines. II. Anhydrous Theobromine". Acta Crystallographica Section C Crystal Structure Communications. 54 (12): 1980–1983. Bibcode:1998AcCrC..54.1980F. doi:10.1107/S0108270198009469.
- ^ a b c d e "Theobromine". PubChem, US National Library of Medicine. 27 August 2022. Retrieved 3 September 2022.
- ^ a b c d e f g Smit HJ (2011). "Theobromine and the Pharmacology of Cocoa". Methylxanthines. Handbook of Experimental Pharmacology. Vol. 200. pp. 201–234. doi:10.1007/978-3-642-13443-2_7. ISBN 978-3-642-13442-5. PMID 20859797.
- ^ "Theophylline". PubChem, US National Library of Medicine. 26 August 2023. Retrieved 2 September 2023.
- ^ Baer DM, Pinkston EM (1997). Environment and Behavior. Westview Press. p. 200. ISBN 978-0813331591.
- ^ von Bibra E, Ott J (1995). Plant Intoxicants: A Classic Text on the Use of Mind-Altering Plants. Inner Traditions / Bear & Co. pp. 67–. ISBN 978-0-89281-498-5. Archived from the original on 2019-09-18. Retrieved 2015-12-12.
- ^ Woskresensky A (1842). "Über das Theobromin". Liebigs Annalen der Chemie und Pharmacie. 41: 125–127. doi:10.1002/jlac.18420410117. Archived from the original on 2016-06-10. Retrieved 2015-12-12.
- ^ Thorpe TE (1902). Essays in Historical Chemistry. The MacMillan Company.
- ^ Fischer, Emil (1882). "Umwandlung des Xanthin in Theobromin und Caffein". Berichte der Deutschen Chemischen Gesellschaft. 15 (1): 453–456. doi:10.1002/cber.18820150194. Archived (PDF) from the original on 2019-05-05. Retrieved 2019-09-09.
- ^ Fischer E (1882). "Über Caffein, Theobromin, Xanthin und Guanin". Justus Liebigs Annalen der Chemie. 215 (3): 253–320. doi:10.1002/jlac.18822150302.
- ^ "-ine". The American Heritage Dictionary of the English Language, Fourth Edition. Houghton Mifflin Company. 2004. ISBN 978-0-395-71146-0. Archived from the original on 2016-03-03. Retrieved 2007-02-23.
- ^ Bennett AW, Bealer BK (2002). The World of Caffeine: The Science and Culture of the World's Most Popular Drug. Routledge, New York. ISBN 978-0-415-92723-9. (note: the book incorrectly states that the name "theobroma" is derived from Latin)
- ^ "AmerMed cocoa extract with 10% theobromine". AmerMed. Archived from the original on 2015-08-11. Retrieved 2008-04-13.
- ^ "USDA Nutrient database, entries for milk chocolate". Archived from the original on 2017-07-08. Retrieved 2012-12-29.
- ^ "USDA Nutrient database, entries for dark chocolate". Retrieved 2012-11-07.
- ^ Kuribara H, Tadokoro S (April 1992). "Behavioral effects of cocoa and its main active compound theobromine: evaluation by ambulatory activity and discrete avoidance in mice". Arukoru Kenkyu to Yakubutsu Izon = Japanese Journal of Alcohol Studies & Drug Dependence. 27 (2): 168–179. PMID 1586288.
- ^ "Theobromine content in plant sources". Dr. Duke's Phytochemical and Ethnobotanical Databases, United States Department of Agriculture. 6 February 2019. Archived from the original on 8 May 2019. Retrieved 9 March 2019.
- ^ Crown PL, Emerson TE, Gu J, Hurst WJ, Pauketat TR, Ward T (August 2012). "Ritual Black Drink consumption at Cahokia". Proc. Natl. Acad. Sci. U.S.A. 109 (35): 13944–9. doi:10.1073/pnas.1208404109. PMC 3435207. PMID 22869743.
- ^ Schuster J, Mitchell ES (March 2019). "More than just caffeine: psychopharmacology of methylxanthine interactions with plant-derived phytochemicals". Progress in Neuro-Psychopharmacology & Biological Psychiatry. 89: 263–274. doi:10.1016/j.pnpbp.2018.09.005. PMID 30213684. S2CID 52274913.
- ^ Prance G, Nesbitt M (2004). The Cultural History of Plants. New York: Routledge. pp. 137, 175, 178–180. ISBN 978-0-415-92746-8.
- ^ "FoodData Central". fdc.nal.usda.gov.
- ^ Craig WJ, Nguyen TT (1984). "Caffeine and theobromine levels in cocoa and carob products". Journal of Food Science. 49 (1): 302–303. doi:10.1111/j.1365-2621.1984.tb13737.x.
Mean theobromine and caffeine levels respectively, were 0.695 mg/g and 0.071 mg/g in cocoa cereals; 1.47 mg/g and 0.152 mg/g in chocolate bakery products; 1.95 mg/g and 0.138 mg/g in chocolate toppings; 2.66 mg/g and 0.208 mg/g in cocoa beverages; 0.621 mg/g and 0.032 mg/g in chocolate ice creams; 0.226 mg/g and 0.011 mg/g in chocolate milks; 74.8 mg/serving and 6.5 mg/serving in chocolate puddings.... Theobromine and caffeine levels in carob products ranged from 0–0.504 mg/g and 0-0.067 mg/g, respectively.
- ^ Ashihara H, Yokota T, Crozier A (2013). "Biosynthesis and catabolism of purine alkaloids". New Light on Alkaloid Biosynthesis and Future Prospects. Vol. 68. pp. 111–138. doi:10.1016/B978-0-12-408061-4.00004-3. ISBN 9780124080614.
{{cite book}}:|journal=ignored (help) - ^ "Caffeine". The Pharmacogenetics and Pharmacogenomics Knowledge Base. Archived from the original on 2010-11-24. Retrieved 2011-01-08.
- ^ Cornish HH, Christman AA (September 1957). "A study of the metabolism of theobromine, theophylline, and caffeine in man". The Journal of Biological Chemistry. 228 (1): 315–323. doi:10.1016/S0021-9258(18)70714-X. PMID 13475320.
- ^ Gates S, Miners JO (March 1999). "Cytochrome P450 isoform selectivity in human hepatic theobromine metabolism". British Journal of Clinical Pharmacology. 47 (3): 299–305. doi:10.1046/j.1365-2125.1999.00890.x. PMC 2014222. PMID 10215755.
- ^ Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, et al. (1 December 1996). "Absorption rate of methylxanthines following capsules, cola and chocolate". European Journal of Clinical Pharmacology. 51 (3–4): 319–325. doi:10.1007/s002280050205. PMID 9010706. S2CID 8405909.
- ^ Essayan DM (November 2001). "Cyclic nucleotide phosphodiesterases". The Journal of Allergy and Clinical Immunology. 108 (5): 671–680. doi:10.1067/mai.2001.119555. PMID 11692087.
- ^ Hardman J, Limbird L, eds. (2001). Goodman & Gilman's the pharmacological basis of therapeutics, 10th ed. New York: McGraw-Hill. p. 745. ISBN 978-0-07-135469-1.
- ^ "Theobromine". DrugBank.ca. Archived from the original on 13 November 2018. Retrieved 3 November 2018.
- ^ "3,7-Dimethylxanthine (Theobromine)". Toxnet, US National Library of Medicine. 1 December 2017. Archived from the original on 7 October 2018. Retrieved 13 November 2018.
- ^ Latif R (March 2013). "Chocolate/cocoa and human health: a review". The Netherlands Journal of Medicine. 71 (2): 63–68. PMID 23462053.
- ^ "Chocolate – Toxicology – Merck Veterinary Manual". Archived from the original on 12 July 2014. Retrieved 23 December 2017.
- ^ Gwaltney-Brant S. "Chocolate Toxicosis in Animals". Merck Veterinary Manual. Merck & Co., Inc. Retrieved 24 December 2023.
- ^ Gwaltney-Brant S. "Chocolate intoxication" (PDF). aspcapro.org. ASPCA. Archived from the original (PDF) on 8 February 2017. Retrieved 23 December 2017.
- ^ a b "The Poisonous Chemistry of Chocolate". Wired. 14 February 2013. Archived from the original on 8 February 2017. Retrieved 12 March 2017.
- ^ "HEALTH WATCH: How to Avoid a Canine Chocolate Catastrophe!". The News Letter. Belfast, Northern Ireland. 2005-03-01.
