Rutherford scattering experiments

The Rutherford scattering experiments were a landmark series of experiments by which scientists learned that every atom has a nucleus where all of its positive charge and most of its mass is concentrated. They deduced this after measuring how an alpha particle beam is scattered when it strikes a thin metal foil. The experiments were performed between 1906 and 1913 by Hans Geiger and Ernest Marsden under the direction of Ernest Rutherford at the Physical Laboratories of the University of Manchester. The most crucial of these experiments was performed in 1909, being the one where they discovered angles of scattering greater than 90 degrees.
The physical phenomenon was explained by Ernest Rutherford in a classic 1911 paper that eventually lead to the widespread use of scattering in particle physics to study subatomic matter. Rutherford scattering or Coulomb scattering is the elastic scattering of charged particles by the Coulomb interaction. The paper also initiated the development of the planetary Rutherford model of the atom and eventually the Bohr model.
Rutherford scattering is now exploited by the materials science community in an analytical technique called Rutherford backscattering.
Summary
[edit]Thomson's model of the atom
[edit]
The prevailing model of atomic structure before Rutherford's experiments was devised by J. J. Thomson. Thomson had discovered the electron through his work on cathode rays[1] and concluded that an electric current is electrons flowing from one atom to an adjacent atom in a series. When no electric current is in effect, electrons remain embedded within atoms. To explain why atoms are electrically neutral, he proposed the existence of a commensurate amount of positive charge that balanced the negative charge of the electrons. Having no idea what the source of this positive charge was, he tentatively proposed that the positive charge was everywhere in the atom, adopting a spherical shape for simplicity.[2] Thomson thought of the positive sphere as being akin to a liquid in that the electrons could move about in it, their arrangement and movements being determined by the balance of electrostatic forces.
Thomson was not quite satisfied with this simplistic idea and hoped to dispense with it as he refined his model.[3] Thomson was never able to develop a complete and stable model that could predict any of the other known properties of the atom, such as emission spectra and valencies.[4] The Japanese scientist Hantaro Nagaoka rejected Thomson's model on the grounds that opposing charges cannot penetrate each other.[5] He proposed instead that electrons orbit the positive charge like the rings around Saturn.[6] However this model was also known to be unstable.[7]: 303
Alpha particles and the Thomson atom
[edit]An alpha particle is an invisible positively-charged particle of matter that is spontaneously emitted from certain radioactive elements. Alpha particles can be detected with the use of phosphorescent screens, photographic plates, or electrodes. Rutherford discovered them in 1899.[8] In 1906, by studying how alpha particle beams are deflected by magnetic and electric fields, he deduced that they were essentially helium atoms stripped of their electrons because they had the same charge-to-mass ratio and atomic weight.[9] Protons and neutrons had yet to be discovered, so Rutherford knew nothing about the structure of alpha particles.
Thomson's model was consistent with the experimental evidence available at the time. Thomson studied beta particle scattering which showed small angle deflections modeled as interaction of the particle with many atoms in succession. Each interaction of the particle with the electrons of the atom and the positive background sphere would lead to a tiny deflection, but many such collisions could add up.[7]: 274 The scattering of alpha particles was expected to be similar. Rutherford's team would show the scattering model to be incorrect because the model of the atom was incorrect.
Rutherford, Geiger, and Marsden
[edit]Ernest Rutherford was Langworthy Professor of Physics at the Victoria University of Manchester[10]: 188 (now the University of Manchester). He had already received numerous honours for his studies of radiation. He had discovered the existence of alpha rays, beta rays, and gamma rays, and had proved that these were the consequence of the disintegration of atoms. In 1906, he received a visit from a German physicist named Hans Geiger, and was so impressed that he asked Geiger to stay and help him with his research.[11] Ernest Marsden was a physics undergraduate student studying under Geiger.
In 1908, Rutherford sought to independently determine the charge and mass of alpha particles. To do this, he wanted to count the number of alpha particles and measure their total charge; the ratio would give the charge of a single alpha particle. Alpha particles are so tiny as to be individually invisible, but Rutherford knew from work by J S Townsend in 1902 that alpha particles ionize air molecules, and if the air is within a strong electric field, each ion will produce a cascade of ions giving a pulse of electric current. On this principle, Rutherford and Geiger designed a simple counting device which consisted of two electrodes in a glass tube. (See #1908 experiment.) Every alpha particle that passed through the tube would create a pulse of electricity that could be counted. It was an early version of the Geiger counter.[7]: 261
The counter that Geiger and Rutherford built proved unreliable because the alpha particles were being too strongly deflected by their collisions with the molecules of air within the detection chamber. The highly variable trajectories of the alpha particles meant that they did not all generate the same number of ions as they passed through the gas, thus producing erratic readings. This puzzled Rutherford because he had thought that alpha particles were just too heavy to be deflected so strongly. Rutherford asked Geiger to investigate just how far matter could scatter alpha rays.[12]
The experiments they designed involved bombarding a metal foil with a beam of alpha particles to observe how the foil scattered them in relation to its thickness and material. They used a phosphorescent screen to measure the trajectories of the particles. Each impact of an alpha particle on the screen produced a tiny flash of light. Geiger worked in a darkened lab for hours on end, counting these tiny scintillations using a microscope.[13] Rutherford lacked the patience for this work.[14] For the metal foil, they tested a variety of metals, but they favored gold because they could make the foil very thin, as gold is the most malleable metal.[15] As a source of alpha particles, Rutherford's substance of choice was radon, a substance several million times more radioactive than uranium.
The outcome of the experiments
[edit]
Right: What Geiger and Marsden observed was that a small fraction of the alpha particles experienced strong deflection.
They had discovered that the metal foils could scatter some alpha particles in all directions, sometimes more than 90°. This should have been impossible according to Thomson's model; it relied on electron scattering and the electrons are too light to turn the heavier alpha particle to the side. This forced Rutherford to revise the model of the atom. In Rutherford's new model, the positive sphere is at least 10,000 times smaller than what Thomson imagined: it doesn't fill the entire volume of the atom but instead a tiny nucleus, and is surrounded by a cloud of electrons that fills the greater volume of the atom. Per Coulomb's law, this means that the positive sphere produces a much more intense electric field at its surface. This nucleus of positive charge also carries all the mass that is not carried by the electrons, which is to say nearly all of it. Thus anchored by its high mass, the nucleus can deflect a passing alpha particle very strongly if said particle comes close enough.
Legacy
[edit]For the most part Rutherford's atomic model was completely ignored immediately after it was published. The probability techniques he used and confusing collection of observations involved were not immediately compelling.[7]: 304 This all changed once Niels Bohr arrived as a post-doctoral student in Manchester at Rutherford's invitation. He dropped his work on the Thomson model in favor of Rutherford's nuclear model, developing the Rutherford–Bohr model over the next several years.
Hantaro Nagaoka, who had once proposed a Saturnian model of the atom, wrote to Rutherford from Tokyo in 1911: "I have been struck with the simpleness of the apparatus you employ and the brilliant results you obtain."[16]
The astronomer Arthur Eddington called Rutherford's discovery the most important scientific achievement since Democritus proposed the atom ages earlier.[17] Rutherford has since been hailed as "the father of nuclear physics".
In a lecture delivered on October 15, 1936 at Cambridge University,[18][19] Rutherford commented on his reaction to the results of the 1909 experiment (see below for details):
Then I remember two or three days later Geiger coming to me in great excitement and saying, "We have been able to get some of the α-particles coming backwards...". It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration, I realized that this scattering backward must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greater part of the mass of the atom was concentrated in a minute nucleus. It was then that I had the idea of an atom with a minute massive centre, carrying a charge.[20]
According to classical Newtonian physics, Rutherford's model of the atom is impossible. Accelerating charged particles radiate electromagnetic waves, so an electron orbiting an atomic nucleus in theory would spiral into the nucleus as it loses energy. Obviously this was not happening, which meant that the classic laws of physics do not apply at the atomic scale. This eventually led Niels Bohr to incorporate quantum mechanics into the model of the atom. These developments came roughly at the same time Albert Einstein produced his theory of general relativity, which showed that the classic laws of physics do not apply at the cosmic scale either.
The experiments
[edit]Alpha particle scattering: 1906 and 1908 experiments
[edit]In 1906, Rutherford noticed that alpha particles passing through sheets of mica were deflected by the sheets by as much as 2 degrees. Rutherford placed a radioactive source in a sealed tube ending with a narrow slits followed by a photographic plate. Half of the slit was covered by a thin layer of mica. A magnetic field around the tube was altered every 10 minutes to reject the effect of beta rays, known to be sensitive to magnetic fields.[21] The tube was evacuated to different amounts and a series of images recorded. At the lowest pressure the image of the open slit was clear, while images of the mica covered slit or the open slit at higher pressures was fuzzy. Rutherford explained these results as alpha-particle scattering[7]: 260 in a paper published in 1906.[22]
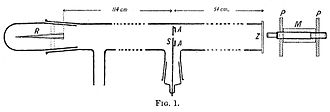
A 1908 paper by Geiger, On the Scattering of α-Particles by Matter, describes the following experiment. He constructed a long glass tube, nearly two meters in length. At one end of the tube was a quantity of "radium emanation" (R) that served as a source of alpha particles. The opposite end of the tube was covered with a phosphorescent screen (Z). In the middle of the tube was a 0.9 mm-wide slit. The alpha particles from R passed through the slit and created a glowing patch of light on the screen. A microscope (M) was used to count the scintillations on the screen and measure their spread. Geiger pumped all the air out of the tube so that the alpha particles would be unobstructed, and they left a neat and tight image on the screen that corresponded to the shape of the slit. Geiger then allowed some air in the tube, and the glowing patch became more diffuse. Geiger then pumped out the air and placed some metal foil over the slit at AA, either gold or aluminium. This too caused the patch of light on the screen to become more spread out. This experiment demonstrated that both air and solid matter could markedly scatter alpha particles.[23]
Alpha particle reflection: the 1909 experiment
[edit]The results of the initial alpha particle scattering experiments were confusing. The angular spread of the particle on the screen varied greatly with the shape of the apparatus and its internal pressure. Rutherford suggested that Ernest Marsden, a physics undergraduate student studying under Geiger, should look for diffusely reflected or back-scattered alpha particles, even though these were not expected. Marsden's first crude reflector got results, so Geiger helped him create a more sophisticated apparatus. They were able to demonstrate that 1 in 8000 alpha particle collisions were diffuse reflections. Although this fraction was small, it was much larger than what the Thomson model of the atom could explain.[7]: 264 This lead to the critical experiment was published in 1909.
In a 1909 paper, On a Diffuse Reflection of the α-Particles,[24] Geiger and Marsden described the experiment by which they proved that alpha particles can indeed be scattered by more than 90°. In their experiment, they prepared a small conical glass tube (AB) containing "radium emanation" (radon), "radium A" (actual radium), and "radium C" (bismuth-214); its open end sealed with mica. This was their alpha particle emitter. They then set up a lead plate (P), behind which they placed a fluorescent screen (S). The tube was held on the opposite side of plate, such that the alpha particles it emitted could not directly strike the screen. They noticed a few scintillations on the screen because some alpha particles got around the plate by bouncing off air molecules. They then placed a metal foil (R) to the side of the lead plate. They tested with lead, gold, tin, aluminum, copper, silver, iron, and platinum. They pointed the tube at the foil to see if the alpha particles would bounce off it and strike the screen on the other side of the plate, and observed an increase in the number of scintillations on the screen. Counting the scintillations, they observed that metals with higher atomic mass, such as gold, reflected more alpha particles than lighter ones such as aluminium.[24]
Geiger and Marsden then wanted to estimate the total number of alpha particles that were being reflected. The previous setup was unsuitable for doing this because the tube contained several radioactive substances (radium plus its decay products) and thus the alpha particles emitted had varying ranges, and because it was difficult for them to ascertain at what rate the tube was emitting alpha particles. This time, they placed a small quantity of radium C (bismuth-214) on the lead plate, which bounced off a platinum reflector (R) and onto the screen. They found that only a tiny fraction of the alpha particles that struck the reflector bounced onto the screen (in this case, 1 in 8,000).[24]
Dependence on foil material and thickness: the 1910 experiment
[edit]
A 1910 paper[25] by Geiger, The Scattering of the α-Particles by Matter, describes an experiment by which he sought to measure how the most probable angle through which an alpha particle is deflected varies with the material it passes through, the thickness of said material, and the velocity of the alpha particles. He constructed an airtight glass tube from which the air was pumped out. At one end was a bulb (B) containing "radium emanation" (radon-222). By means of mercury, the radon in B was pumped up the narrow glass pipe whose end at A was plugged with mica. At the other end of the tube was a fluorescent zinc sulfide screen (S). The microscope which he used to count the scintillations on the screen was affixed to a vertical millimeter scale with a vernier, which allowed Geiger to precisely measure where the flashes of light appeared on the screen and thus calculate the particles' angles of deflection. The alpha particles emitted from A was narrowed to a beam by a small circular hole at D. Geiger placed a metal foil in the path of the rays at D and E to observe how the zone of flashes changed. He tested gold, tin, silver, copper, and aluminium. He could also vary the velocity of the alpha particles by placing extra sheets of mica or aluminium at A.[25]
From the measurements he took, Geiger came to the following conclusions:
- the most probable angle of deflection increases with the thickness of the material
- the most probable angle of deflection is proportional to the atomic mass of the substance
- the most probable angle of deflection decreases with the velocity of the alpha particles
Rutherford's Structure of the Atom paper (1911)
[edit]
Considering the results of the above experiments, Rutherford published a landmark paper in 1911 titled "The Scattering of α and β Particles by Matter and the Structure of the Atom" wherein he showed that single scattering from a very small and intense electric charge predicts primarily small-angle scattering with small but measurable amounts of backscattering.[26] For the purpose of his mathematical calculations he assumed this central charge was positive, but he admitted he could not prove this and that he had to wait for other experiments to develop his theory.
Rutherford developed a mathematical equation that modeled how the foil should scatter the alpha particles if all the positive charge and most of the atomic mass was concentrated in a point at the center of an atom.

- s = the number of alpha particles falling on unit area at an angle of deflection Φ
- r = distance from point of incidence of α rays on scattering material
- X = total number of particles falling on the scattering material
- n = number of atoms in a unit volume of the material
- t = thickness of the foil
- qn = positive charge of the atomic nucleus
- qa = positive charge of the alpha particles
- m = mass of an alpha particle
- v = velocity of the alpha particle
From the scattering data, Rutherford estimated the central charge qn to be about +100 units[27]
Rutherford's paper does not discuss any electron arrangement beyond discussions on the scattering from JJ Thomson's plum pudding model and from Hantaro Nagaoka's Saturnian model.[7]: 303 He shows that the scattering results predicted by Thomson's model are also explained by single scattering, but that Thomson's model does not explain large angle scattering. He says that Nagaoka's model, having a compact charge, would agree with the scattering data. The Saturnian model had previously been rejected on other grounds. The so-called Rutherford model of the atom with orbiting electrons was not proposed by Rutherford in the 1911 paper.[7]: 304
Confirming the scattering theory: the 1913 experiment
[edit]In a 1913 paper, The Laws of Deflexion of α Particles through Large Angles,[28] Geiger and Marsden describe a series of experiments by which they sought to experimentally verify the above equation that Rutherford developed. Rutherford's equation predicted that the number of scintillations per minute s that will be observed at a given angle Φ should be proportional to:
- cosec4Φ/2
- thickness of foil t
- magnitude of the square of central charge Qn
- 1/(mv2)2
Their 1913 paper describes four experiments by which they proved each of these four relationships.
To test how the scattering varied with the angle of deflection (i.e. if s ∝ csc4Φ/2). Geiger and Marsden built an apparatus that consisted of a hollow metal cylinder mounted on a turntable. Inside the cylinder was a metal foil (F) and a radiation source containing radon (R), mounted on a detached column (T) which allowed the cylinder to rotate independently. The column was also a tube by which air was pumped out of the cylinder. A microscope (M) with its objective lens covered by a fluorescent zinc sulfide screen (S) penetrated the wall of the cylinder and pointed at the metal foil. They tested with silver and gold foils. By turning the table, the microscope could be moved a full circle around the foil, allowing Geiger to observe and count alpha particles deflected by up to 150°. Correcting for experimental error, Geiger and Marsden found that the number of alpha particles that are deflected by a given angle Φ is indeed proportional to csc4Φ/2.[28]
Geiger and Marsden then tested how the scattering varied with the thickness of the foil (i.e. if s ∝ t). They constructed a disc (S) with six holes drilled in it. The holes were covered with metal foil (F) of varying thickness, or none for control. This disc was then sealed in a brass ring (A) between two glass plates (B and C). The disc could be rotated by means of a rod (P) to bring each window in front of the alpha particle source (R). On the rear glass pane was a zinc sulfide screen (Z). Geiger and Marsden found that the number of scintillations that appeared on the zinc sulfide screen was indeed proportional to the thickness as long as said thickness was small.[28]
Geiger and Marsden reused the above apparatus to measure how the scattering pattern varied with the square of the nuclear charge (i.e. if s ∝ Qn2). Geiger and Marsden did not know what the positive charge of the nucleus of their metals were (they had only just discovered the nucleus existed at all), but they assumed it was proportional to the atomic weight, so they tested whether the scattering was proportional to the atomic weight squared. Geiger and Marsden covered the holes of the disc with foils of gold, tin, silver, copper, and aluminum. They measured each foil's stopping power by equating it to an equivalent thickness of air. They counted the number of scintillations per minute that each foil produced on the screen. They divided the number of scintillations per minute by the respective foil's air equivalent, then divided again by the square root of the atomic weight (Geiger and Marsden knew that for foils of equal stopping power, the number of atoms per unit area is proportional to the square root of the atomic weight). Thus, for each metal, Geiger and Marsden obtained the number of scintillations that a fixed number of atoms produce. For each metal, they then divided this number by the square of the atomic weight, and found that the ratios were more or less the same. Thus they proved that s ∝ Qn2.[28]
Finally, Geiger and Marsden tested how the scattering varied with the velocity of the alpha particles (i.e. if s ∝ 1/v4). Using the same apparatus again, they slowed the alpha particles by placing extra sheets of mica in front of the alpha particle source. They found that, within the range of experimental error, that the number of scinitillations was indeed proportional to 1/v4.[28]
Postive charge on nucleus: 1913
[edit]In his 1911 paper (see above), Rutherford assumed that the central charge of the atom was positive, but a negative charge would have fitted his scattering model just as well.[29] In a 1913 paper,[30] Rutherford declared that the "nucleus" (as he now called it) was indeed positively charged, based on the result of experiments exploring the scattering of alpha particles in various gases.
In 1917, Rutherford and his assistant William Kay began exploring the passage of alpha particles through gases such as hydrogen and nitrogen. In this experiment, they shot a beam of alpha particles through hydrogen, and they carefully placed their detector—a zinc sulfide screen—just beyond the range of the alpha particles, which were absorbed by the gas. They nonetheless picked up charged particles of some sort causing scintillations on the screen. Rutherford interpreted this as alpha particles knocking the hydrogen nuclei forwards in the direction of the beam, not backwards.[29]
Atomic model in Rutherford's crucial 1911 paper
[edit]Rutherford begins his 1911 paper[26] with a discussion of Thomson's results on scattering of beta particles, a form of radioactivity that results in high velocity electrons. Thomson's model had electrons circulating inside of a sphere of positive charge. Rutherford highlights the need for compound or multiple scattering events: the deflections predicted for each collision are much less than one degree. He then proposes a model which will produce large deflections on a single encounter: place all of the positive charge at the center of the sphere and ignore the electron scattering as insignificant. The concentrated charge will explain why most alpha particles do not scatter at all – they miss the charge altogether – and yet particles that do hit the center scatter through large angles.[7]: 285
Maximum nuclear size estimate
[edit]Rutherford begins his analysis by considering a head-on collision between the alpha particle and atom. This will establish the minimum distance between them, a value which will be used throughout his calculations.
Assuming there are no external forces and that initially the alpha particles are far from the nucleus, the inverse-square law between the charges on the alpha particle and nucleus gives the potential energy gained by the particle as it approaches the nucleus. For head-on collisions between alpha particles and the nucleus, all the kinetic energy of the alpha particle is turned into potential energy and the particle stops and turns back.

Where the particle stops, a distance the potential energy matches the original kinetic energy:[31]: 620 [32]: 320
Rearranging:
For an alpha particle:
- m (mass) = 6.64424×10−27 kg = 3.7273×109 eV/c2
- q1 (for helium) = 2 × 1.6×10−19 C = 3.2×10−19 C
- q2 (for gold) = 79 × 1.6×10−19 C = 1.27×10−17 C
- v (initial velocity) = 2×107 m/s (for this example)
The distance from the alpha particle to the center of the nucleus (rmin) at this point is an upper limit for the nuclear radius. Substituting these in gives the value of about 2.7×10−14 m, or 27 fm. (The true radius is about 7.3 fm.) The true radius of the nucleus is not recovered in these experiments because the alphas do not have enough energy to penetrate to more than 27 fm of the nuclear center, as noted, when the actual radius of gold is 7.3 fm.

Rutherford's 1911 paper[26] started with a slightly different formula suitable for head-on collision with a sphere of positive charge: Rutherford used as the turning point distance called rmin above and is the radius of the atom. The first term is the Coulomb repulsion used above. This form assumes the alpha particle could penetrate the positive charge. At the time of Rutherford's paper, Thomson's plum pudding model proposed a positive charge with the radius of an atom, thousands of times larger than the rmin found above. Fig. 1 shows how concentrated this potential is compared to the size of the atom.
Single scattering from heavy nuclei
[edit]From his results for a head on collision, Rutherford knows that alpha particle scattering occurs close to the center of an atom, at a radius 10,000 times smaller than the atom. Therefore he ignores the effect of "negative electricity". Furthermore he begins by assuming no energy loss in the collision, that is he ignores the recoil of the target atom. He will revisit each of these issues later in his paper.[26]: 672

Under these conditions, the alpha particle and atom interact through a central force, a physical problem studied first by Isaac Newton.[33] For the inverse square law like the Coulomb force, a detailed theory was developed under the name of the Kepler problem.[34]: 76 Thus Rutherford proposed that the alpha particle will take a hyperbolic trajectory in the repulsive force near the center of the atom as shown in Fig. 1. He derives his scattering formula by starting with conservation of angular momentum. When the particle of mass and velocity is far from the atom, its angular momentum around the center of the atom will be where is the perpendicular distance between the incoming particle path and the atom, now called the impact parameter. At the point of closest approach, labeled A in the Fig. 1, the angular momentum will be . Thus Rutherford (in a slightly different notation, Rutherford's SA is here) equates Next he uses conservation of energy at these two points: The left hand side and the first term on the right hand side are the kinetic energies of the particle at the two points; the last term is the potential energy due to the Coulomb force between the particle and atom at the point of closest approach (A). Ne is the nuclear charge of the atom and E is the charge of the alpha particle, both expressed in electrostatic units. Rutherford valued e at 4.65×10−10 esu.
Next Rutherford rearranges the energy equation and divides by half the mass: In the same step he implicitly introduces a variable containing the non-geometrical physical constants of the problem: (This value is the closest approach that Rutherford estimated as earlier in the paper.)

Having assumed an inverse square law force between the alpha particle and the compact massive charge, Rutherford can use known results from Newtonian gravity, which also an inverse square law.[35]: 151 The appropriate orbit will be hyperbolic since the alpha particle has kinetic energy far from the charge. The hyperbola can be written in polar coordinates with the origin between the two foci as where is the semiminor axis and is the eccentricity. As the alpha particle scatters, the angle swings between two extreme values, These angles correspond to values where the denominator is zero: Rutherford's deflection angle is the same as in his paper he describes the reciprocal of this formula as "the eccentricity is " (see secant).
This eccentricity is also geometrically related to the point of closest approach, and the impact parameter . Rutherford uses trigonometry to connect the scattering angle to these parameters. The atom, at point S in his diagram, is at one focus (a distance SO from the origin) and the turning point at point A is the apse of the hyperbola (a distance OA). The ratio of the focal distance to apse in a hyperbola is the eccentricity: or in terms of the diagram: The distance SO is the hypotenuse of the right triangle with opposite the impact parameter : The distance can then be written in terms of scattering angle and impact parameter: when a half-angle formula is applied.
Dividing the conservation of angular momentum equation by and squaring gives another equation involving the velocity at closest approach, squared: Combining energy and angular momentum equations eliminates the velocity : Using the previous equation for and solving for relates the physical and geometrical variables: The scattering angle of the particle is so his relationship between scattering angle and impact parameter becomes:

Rutherford gives some illustrative values as shown in this table:[26]: 673
| 10 | 5 | 2 | 1 | 0.5 | 0.25 | 0.125 | |
| 5.7° | 11.4° | 28° | 53° | 90° | 127° | 152° |
Rutherford's approach to this scattering problem remains a standard treatment in textbooks[35]: 151 [36]: 240 [37]: 400 on classical mechanics.
Intensity vs angle
[edit]
To compare to experiments the relationship between impact parameter and scattering angle needs to be converted to probability versus angle. The scattering cross section gives the relative intensity by angles:[34]: 81
In classical mechanics, the scattering angle Θ is uniquely determined the initial kinetic energy of the incoming particles and the impact parameter p.[34]: 82 Therefore, the number of particles scattered into an angle between Θ and Θ + dΘ must be the same as the number of particles with associated impact parameters between p and p + dp. For an incident intensity I, this implies: Thus the cross section depends on scattering angle as: Using the impact parameter as a function of angle, p(Θ), from the single scattering result above produces the Rutherford scattering cross section:[34]: 84

This formula predicted the results that Geiger measured in the coming year. The scattering probability into small angles greatly exceeds the probability in to larger angles, reflecting the tiny nucleus surrounded by empty space. However, for rare close encounters, large angle scattering occurs with just a single target.[38]: 19
At the end of his development of the cross section formula, Rutherford emphasizes that the results apply to single scattering and thus require measurements with thin foils. For thin foils the amount of scattering is proportional to the foil thickness in agreement with Geiger's measurements.[26]
Comparison to JJ Thomson's results
[edit]At the time of Rutherford's paper, JJ Thomson was the "undisputed world master in the design of atoms".[7]: 296 Rutherford needed to compare his new approach to Thomson's. Thomson's model, presented in 1910,[39] modeled the electron collisions with hyperbolic orbits from his 1906 paper[40] combined with a factor for the positive sphere. Multiple resulting small deflections compounded using a random walk.[7]: 277
In his paper Rutherford emphasized that single scattering alone could account for Thomson's results if the positive charge were concentrated in the center. Rutherford computes the probability of single scattering from a compact charge and demonstrates that it is 3 times larger than Thomson's multiple scattering probability. Rutherford completes his analysis including the effects of density and foil thickness, then concludes that thin foils are governed by single scattering, not multiple scattering.[7]: 298
Later analysis showed Thomson's scattering model could not account for large scattering. The maximum angular deflection from electron scattering or from the positive sphere each come to less than 0.02°; even many such scattering events compounded would result in less than a one degree average deflection and a probability of scattering through 90° of less than one in 103500.[41]: 106
Target recoil
[edit]Rutherford's analysis assumed that alpha particle trajectories turned at the center of the atom but the exit velocity was not reduced. This is equivalent to assuming that the concentrated charge at the center had infinite mass or was anchored in place. Rutherford discusses the limitations of this assumption by comparing scattering from lighter atoms like aluminum with heavier atoms like gold. If the concentrated charge is lighter it will recoil from the interaction, gaining momentum while the alpha particle loses momentum and consequently slows down.
Modern treatments analyze this type of Coulomb scattering in the center of mass reference frame. The six coordinates of the two particles (also called "bodies") are converted into three relative coordinates between the two particles and three center-of-mass coordinates moving in space (called the lab frame). The interaction only occurs in the relative coordinates, giving an equivalent one-body problem[34]: 58 just as Rutherford solved, but with different interpretations for the mass and scattering angle.
Rather than the mass of the alpha particle, the more accurate formula including recoil uses reduced mass: For Rutherford's alpha particle scattering from gold, with mass of 197, the reduced mass is very close to the mass of the alpha particle: For lighter aluminum, with mass 27, the effect is greater: a 13% difference in mass. Rutherford notes this difference and suggests experiments be performed with lighter atoms.
The second effect is a change in scattering angle. The angle in the relative coordinate system or center of mass frame needs to be converted to an angle in the lab frame.[34]: 85 In the lab frame, denoted by a subscript L, the scattering angle for a general central potential is For a heavy particle like gold used by Rutherford, and at almost all angles we can neglect this factor: the lab and relative angles are the same, .
The change in scattering angle alters the formula for differential cross-section needed for comparsion to experiment. For any central potential, the differential cross-section in the lab frame is related to that in the center-of-mass frame by[34]: 88 where
Reception
[edit]
Rutherford's 1911 paper caused little reaction.[10]: 192 The paper was primarily about alpha particle scattering in an era before particle scattering was a primary tool for physics. For example the first results from a cloud chamber, by C.T.R. Wilson shows alpha particle scattering and also appeared in 1911.[42][7]: 302
Rutherford explicitly ignores the electrons, only mentioning Hantaro Nagaoka's Saturnian model of electrons orbiting a tiny "sun", a model that had been previously rejected as mechanically unstable. By ignoring the electrons Rutherford also ignores any potential implications for atomic spectroscopy for chemistry. [10]: 302 Niels Bohr joined Rutherford in 1912 and developed an his revolutionary new electronic atom model over the next few years.
Newtonian mechanics for scattering
[edit]Rutherford's 1911 paper used the hyperbolic orbit solutions for inverse-square central force problem developed since the time of Isaac Newton and available in textbooks in Rutherford's era, e.g. ref. [43]: 86 Rutherford then shows how this orbit relates the particle impact parameter to deflection angle and how that can be used to predict the experimental intensity versus angle data for his model of the atom. The relation between impact parameter and deflection angle can also be derived using Newtonian mechanics directly.
This section uses the following variables and values for an alpha particle passing by the nucleus of a gold atom:
- qg = positive charge of the gold atom = 79 e = 1.26×10−17 C
- qa = charge of the alpha particle = 2 e = 3.20×10−19 C
- r = radius of the gold atom = 1.44×10−10 m
- v = speed of the alpha particle = 1.53×107 m/s
- m = mass of the alpha particle = 6.64×10−27 kg
- k = Coulomb constant = 8.987×109 N·m2/C2
The scattering geometry is shown in this diagram[44][45]
The impact parameter b is the distance between the alpha particle's initial trajectory and a parallel line that goes through the nucleus. Smaller values of b bring the particle closer to the atom so it feels more deflection force resulting in a larger deflection angle θ. The goal is to find the relationship between b and the deflection angle.
The alpha particle's path is a hyperbola and the net change in momentum runs along the axis of symmetry. From the geometry in the diagram and the magnitude of the initial and final momentum vectors, , the magnitude of can be related to the deflection angle:[45]: 111
A second formula for involving b will give the relationship to the deflection angle. The net change in momentum can also be found by adding small increments to momentum all along the trajectory using the integral where is the distance between the alpha particle and the center of the nucleus and is its angle from the axis of symmetry. These two are the polar coordinates of the alpha particle at time . Here the Coulomb force exerted along the line between the alpha particle and the atom is and the factor gives that part of the force causing deflection.
The polar coordinates r and φ depend on t in the integral, but they must be related to each other as they both vary as the particle moves. Changing the variable and limits of integration from t to φ makes this connection explicit:[45]: 112
The factor is the reciprocal of the angular velocity the particle. Since the force is only along the line between the particle and the atom, the angular momentum, which is proportional to the angular velocity, is constant: This law of conservation of angular momentum gives a formula for :
Replacing in the integral for ΔP simultaneously eliminates the dependence on r and the integral is well-known:
Applying the trigonometric identity to simplify this result gives the second formula for : Solving for θ as a function of b gives the final result
Evaluating the formula for an impact parameter, b, equal to the radius of a gold nucleus, 7×10−15 m, gives the deflection angle θ as 2.56 radians (147°). Using instead the radius of a gold atom is 1.44×10−10 m results in a tiny deflection angle θ of 0.000325 radians (0.0186°).[46]
See also
[edit]- Atomic theory
- Rutherford backscattering spectroscopy
- Rutherford scattering
- List of scattering experiments
References
[edit]- ^ J. J. Thomson (1897). "Cathode rays". Philosophical Magazine. 44 (269): 293-316.
- ^ J. J. Thomson (1907). The Corpuscular Theory of Matter, p. 103: "In default of exact knowledge of the nature of the way in which positive electricity occurs in the atom, we shall consider a case in which the positive electricity is distributed in the way most amenable to mathematical calculation, i.e., when it occurs as a sphere of uniform density, throughout which the corpuscles are distributed."
- ^ J. J. Thomson, in a letter to Oliver Lodge dated 11 April 1904, quoted in Davis & Falconer (1997):
"With regard to positive electrification I have been in the habit of using the crude analogy of a liquid with a certain amount of cohesion, enough to keep it from flying to bits under its own repulsion. I have however always tried to keep the physical conception of the positive electricity in the background because I have always had hopes (not yet realised) of being able to do without positive electrification as a separate entity and to replace it by some property of the corpuscles.
When one considers that, all the positive electricity does, on the corpuscular theory, is to provide an attractive force to keep the corpuscles together, while all the observable properties of the atom are determined by the corpuscles one feels, I think, that the positive electrification will ultimately prove superfluous and it will be possible to get the effects we now attribute to it from some property of the corpuscle.
At present I am not able to do this and I use the analogy of the liquid as a way of picturing the missing forces which is easily conceived and lends itself readily to analysis." - ^ Thomson (1907). The Corpuscular Theory of Matter, p. 106: "The general problem of finding how n corpuscles will distribute themselves inside the sphere is very complicated, and I have not succeeded in solving it"
- ^ Daintith & Gjertsen (1999), p. 395
- ^ Hantaro Nagaoka (1904). "Kinetics of a System of Particles illustrating the Line and the Band Spectrum and the Phenomena of Radioactivity". Philosophical Magazine. Series 6. 7 (41): 445–455. doi:10.1080/14786440409463141.
- ^ a b c d e f g h i j k l m Heilbron, John L. (1968). "The Scattering of α and β Particles and Rutherford's Atom". Archive for History of Exact Sciences. 4 (4): 247–307. doi:10.1007/BF00411591. ISSN 0003-9519. JSTOR 41133273.
- ^ Ernest Rutherford (1899). "Uranium Radiation and the Electrical conduction Produced by it" (PDF). Philosophical Magazine. 47 (284): 109–163.
- ^ Ernest Rutherford (1906). "The Mass and Velocity of the α particles expelled from Radium and Actinium". Philosophical Magazine. Series 6. 12 (70): 348–371. doi:10.1080/14786440609463549.
- ^ a b c Pais, Abraham (2002). Inward bound: of matter and forces in the physical world (Reprint ed.). Oxford: Clarendon Press [u.a.] ISBN 978-0-19-851997-3.
- ^ Heilbron (2003), p. 59
- ^ Heilbron (2003)
- ^ Cavendish Laboratory.
- ^ Letter from Ernest Rutherford to Henry Bumstead, 11 July 1908, quoted in Eve (1939)[broken anchor], p. 180: "Geiger is a demon at the work and could count at intervals for a whole night without destroying his equanimity. I damned vigorously after two minutes and retired from the conflict."
- ^ Tibbetts (2007)[broken anchor], p. 127
- ^ Letter from Hantaro Nagaoka to Ernest Rutherford, 22 February 1911. Quoted in Eve (1939), p. 200
- ^ Reeves (2008)
- ^ Report on the Activities of the History of Science Lectures Committee 1936–1947, Whipple Museum Papers, Whipple Museum for the History of Science, Cambridge, C62 i.
The report lists two lectures, on October 8 and 15. The lecture on atomic structure was likely the one delivered on the 15th. - ^ Cambridge University Reporter, 7 October 1936, p. 141
The lecture took place in the lecture room of the Physiological Laboratory at 5 pm. - ^ The Development of the Theory of Atomic Structure (Rutherford 1936). Reprinted in Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936
- ^ Leone, M; Robotti, N; Verna, G (May 2018). "'Rutherford's experiment' on alpha particles scattering: the experiment that never was". Physics Education. 53 (3): 035003. Bibcode:2018PhyEd..53c5003L. doi:10.1088/1361-6552/aaa353. ISSN 0031-9120.
- ^ Rutherford, E. (August 1906). "XIX. Retardation of the α particle from radium in passing through matter". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 12 (68): 134–146. doi:10.1080/14786440609463525. ISSN 1941-5982.
- ^ Geiger (1908)
- ^ a b c Geiger & Marsden (1909)
- ^ a b Geiger (1910)
- ^ a b c d e f Rutherford, E. (1911). "LXXIX. The scattering of α and β particles by matter and the structure of the atom" (PDF). The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 21 (125): 669–688. doi:10.1080/14786440508637080. ISSN 1941-5982.
- ^ Rutherford (1911)
- ^ a b c d e f g Geiger & Marsden (1913)
- ^ a b AIP
- ^ Rutherford & Nuttal (1913)
- ^ "Electrons (+ and -), Protons, Photons, Neutrons, Mesotrons and Cosmic Rays" By Robert Andrews Millikan. Revised edition. Pp. x+642. (Chicago: University of Chicago Press; London: Cambridge University Press, 1947.)
- ^ Cooper, L. N. (1970). "An Introduction to the Meaning and Structure of Physics". Japan: Harper & Row.
- ^ Speiser, David (1996). "The Kepler Problem from Newton to Johann Bernoulli". Archive for History of Exact Sciences. 50 (2): 103–116. doi:10.1007/BF02327155. ISSN 0003-9519.
- ^ a b c d e f g Goldstein, Herbert. Classical Mechanics. United States, Addison-Wesley, 1950.
- ^ a b Hand, Louis N.; Finch, Janet D. (1998-11-13). Analytical Mechanics. doi:10.1017/cbo9780511801662. ISBN 978-0-521-57572-0.
- ^ Fowles, Grant R.; Cassiday, George L. (1993). Analytical mechanics. Saunders golden sunburst series (5th ed.). Fort Worth: Saunders College Pub. ISBN 978-0-03-096022-2.
- ^ Webber, B.R.; Davis, E.A. (February 2012). "Commentary on 'The scattering of α and β particles by matter and the structure of the atom' by E. Rutherford (Philosophical Magazine 21 (1911) 669–688)". Philosophical Magazine. 92 (4): 399–405. Bibcode:2012PMag...92..399W. doi:10.1080/14786435.2011.614643. ISSN 1478-6435.
- ^ Karplus, Martin, and Richard Needham Porter. "Atoms and molecules; an introduction for students of physical chemistry." Atoms and molecules; an introduction for students of physical chemistry (1970).
- ^ Thomson, Joseph J. (1910). "On the scattering of rapidly moving electrified particles". Proceedings of the Cambridge Philosophical Society. 15: 465–471.
- ^ Thomson, J.J. (1906). "LXX. On the number of corpuscles in an atom". The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 11 (66): 769–781. doi:10.1080/14786440609463496. ISSN 1941-5982.
- ^ Beiser, A. (1969). "Perspectives of Modern Physics". Japan: McGraw-Hill.
- ^ On an expansion apparatus for making visible the tracks of ionising particles in gases and some results obtained by its use. Vol. 87. 1912-09-19. pp. 277–292. doi:10.1098/rspa.1912.0081. ISSN 0950-1207.
- ^ Whittaker, E. T.; McCrae, Sir William (1988-12-15). A Treatise on the Analytical Dynamics of Particles and Rigid Bodies (1 ed.). Cambridge University Press. doi:10.1017/cbo9780511608797. ISBN 978-0-521-35883-5.
- ^ "Impact Parameter for Nuclear Scattering". HyperPhysics. Georgia State University. Retrieved 2024-05-30.
- ^ a b c Beiser (1969). Perspectives of Modern Physics, p. 109-113
- ^ "Determining the Impact Parameter". HyperPhysics. Georgia State University. Retrieved 2024-07-05.
Bibliography
[edit]- "Rutherford's Nuclear World: The Story of the Discovery of the Nucleus". American Institute of Physics. Retrieved 2014-10-23.
- "Rutherford scattering". HyperPhysics. Georgia State University. Retrieved 2014-08-13.
- Arthur Beiser (1969). Perspectives of Modern Physics. McGraw-Hill Book Company.
- "Geiger and Marsden". Cavendish Laboratory. Archived from the original on 2014-10-06. Retrieved 2014-07-23.
- John Daintith; Derek Gjertsen (1999). A Dictionary of Scientists. Oxford University Press. ISBN 978-0-19-280086-2.
- Michael Fowler. "Rutherford Scattering". Lecture notes for Physics 252. University of Virginia. Retrieved 2014-07-23.
- Hans Geiger (1908). "On the Scattering of α-Particles by Matter". Proceedings of the Royal Society of London A. 81 (546): 174–177. Bibcode:1908RSPSA..81..174G. doi:10.1098/rspa.1908.0067.
- Hans Geiger; Ernest Marsden (1909). "On a Diffuse Reflection of the α-Particles". Proceedings of the Royal Society of London A. 82 (557): 495–500. Bibcode:1909RSPSA..82..495G. doi:10.1098/rspa.1909.0054.
- Hans Geiger (1910). "The Scattering of the α-Particles by Matter". Proceedings of the Royal Society of London A. 83 (565): 492–504. Bibcode:1910RSPSA..83..492G. doi:10.1098/rspa.1910.0038.
- Hans Geiger; Ernest Marsden (1913). "The Laws of Deflexion of α Particles through Large Angles" (PDF). Philosophical Magazine. Series 6. 25 (148): 604–623. doi:10.1080/14786440408634197.
- John L. Heilbron (January 1968). "The Scattering of α and β Particles and Rutherford's Atom". Archive for History of Exact Sciences. 4 (4): 247–307. doi:10.1007/BF00411591.
- John L. Heilbron (2003). Ernest Rutherford and the Explosion of Atoms. Oxford University Press. ISBN 978-0-19-512378-4.
- John W. Jewett Jr.; Raymond A. Serway (2014). "Early Models of the Atom". Physics for Scientists and Engineers with Modern Physics (9th ed.). Brooks/Cole. p. 1299.
- Joy Manners (2000). Quantum Physics: An Introduction. CRC Press. ISBN 978-0-7503-0720-8.
- Hantaro Nagaoka (1904). "Kinetics of a System of Particles illustrating the Line and the Band Spectrum and the Phenomena of Radioactivity". Philosophical Magazine. Series 6. 7 (41): 445–455. doi:10.1080/14786440409463141.
- Richard Reeves (2008). A Force of Nature: The Frontier Genius of Ernest Rutherford. W. W. Norton & Co. ISBN 978-0-393-07604-2.
- Ernest Rutherford (1899). "Uranium Radiation and the Electrical conduction Produced by it" (PDF). Philosophical Magazine. 47 (284): 109–163.
- Ernest Rutherford (1911). "The Scattering of α and β Particles by Matter and the Structure of the Atom". Philosophical Magazine. Series 6. 21 (125): 669–688. doi:10.1080/14786440508637080.
- Ernest Rutherford (1906). "The Mass and Velocity of the α particles expelled from Radium and Actinium". Philosophical Magazine. Series 6. 12 (70): 348–371. doi:10.1080/14786440609463549.
- Ernest Rutherford (1912). "The origin of β and γ rays from radioactive substances". Philosophical Magazine. Series 6. 24 (142): 453–462. doi:10.1080/14786441008637351.
- Ernest Rutherford; John Mitchell Nuttal (1913). "Scattering of α-Particles by Gases". Philosophical Magazine. Series 6. 26 (154): 702–712. doi:10.1080/14786441308635014.
- Ernest Rutherford (1914). "The Structure of the Atom". Philosophical Magazine. Series 6. 27 (159): 488–498. doi:10.1080/14786440308635117.
- Ernest Rutherford (1938). "Forty Years of Physics". In Needham, Joseph; Pagel, Walter (eds.). Background to Modern Science: Ten Lectures at Cambridge arranged by the History of Science Committee 1936. Cambridge University Press.
- Ernest Rutherford (1913). Radioactive Substances and their Radiations. Cambridge University Press.
- Ernest Rutherford (1936). "Radioactivity and Atomic Structure". Journal of the Chemical Society. 1936: 508–516. doi:10.1039/JR9360000508.
- Joseph J. Thomson (1904). "On the Structure of the Atom: an Investigation of the Stability and Periods of Oscillation of a number of Corpuscles arranged at equal intervals around the Circumference of a Circle; with Application of the Results to the Theory of Atomic Structure". Philosophical Magazine. Series 6. 7 (39): 237. doi:10.1080/14786440409463107.
- Gary Tibbetts (2007). How the Great Scientists Reasoned: The Scientific Method in Action. Elsevier. ISBN 978-0-12-398498-2.
- Arthur Stewart Eve (1939). Rutherford: Being the Life and Letters of the Rt. Hon. Lord Rutherford, O. M. MacMillan.
- Jean Perrin (1910) [1909]. Brownian Movement and Molecular Reality. Translated by F. Soddy. Taylor and Francis.
- E. A. Davis; I. J. Falconer (1997). J. J. Thomson and the Discovery of the Electron. Taylor & Francis. ISBN 0-7484-0720-0.
- Giora Hon; Bernard R. Goldstein (6 September 2013). "J. J. Thomson's plum-pudding atomic model: The making of a scientific myth". Annalen der Physik. 525 (8–9): A129–A133. Bibcode:2013AnP...525A.129H. doi:10.1002/andp.201300732.
- J. J. Thomson (1907). The Corpuscular Theory of Matter. Charles Scribner's Sons.
- Goldstein, Herbert; Poole, Charles; Safko, John (2002). Classical Mechanics (third ed.). Addison-Wesley. ISBN 978-0-201-65702-9.
- Tong, David. "Lectures on Dynamics and Relativity". University of Cambridge. Retrieved 2024-07-14. | Chapter 4 Central forces














































































![{\displaystyle ={\frac {kq_{a}q_{g}}{vb}}\left(\sin \left[{\frac {\pi -\theta }{2}}\right]-\sin \left[-{\frac {\pi -\theta }{2}}\right]\right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e21c790ae221c75d2087c933dddcf2f4edec7be5)


