5-Hydroxytryptophan

| |

| |
| Names | |
|---|---|
| IUPAC name
2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid
| |
| Other names
5-HTP; Oxitriptan
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.193 |
| KEGG | |
| MeSH | 5-Hydroxytryptophan |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H12N2O3 | |
| Molar mass | 220.228 g·mol−1 |
| Density | 1.484 g/mL |
| Melting point | 298 to 300 °C (568 to 572 °F; 571 to 573 K) |
| Boiling point | 520.6 °C (969.1 °F; 793.8 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
5-Hydroxytryptophan (5-HTP), used medically as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.
5-HTP can be manufactured and used as a drug and supplement with the INN oxitriptan. Brand names include Cincofarm, Levothym, Levotonine, Oxyfan, Telesol, Tript-OH, and Triptum. As a drug, it is used in the treatment of depression and for certain other indications.
Production
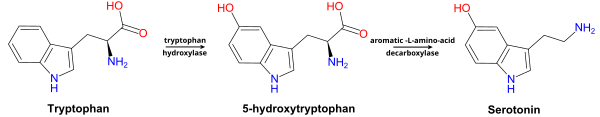
[edit]5-HTP is produced from the amino acid tryptophan through the action of the enzyme tryptophan hydroxylase. Tryptophan hydroxylase is one of the biopterin-dependent aromatic amino acid hydroxylases. Production of 5-HTP is the rate-limiting step in 5-HT (serotonin) synthesis. 5-HTP is normally rapidly converted to 5-HT by amino acid decarboxylase.[1]
Metabolism
[edit]5-HTP is decarboxylated to serotonin (5-hydroxytryptamine or 5-HT) by the enzyme aromatic-L-amino-acid decarboxylase with the help of vitamin B6.[2] This reaction occurs both in nervous tissue and in the liver.[3] 5-HTP crosses the blood–brain barrier,[4] while 5-HT does not. Excess 5-HTP, especially when administered with vitamin B6, is thought to be metabolized and excreted.[5][6]
| 5-HTP | AAAD | Serotonin | |

|

| ||
| PLP | |||

| |||
Dietary sources
[edit]Though 5-HTP is found in food only in insignificant quantities, it is a chemical involved intermediately in the metabolism of tryptophan, an amino acid found in all unfractionated foods, with lower total amino acid content correlating with increased tryptophan absorption.[7]
Use as a medication and supplement
[edit]5-HTP is used medically and as a supplement under the name oxitriptan in the treatment of depression and for certain other indications.
It can be potentiated in combination with a peripherally selective aromatic L-amino acid decarboxylase (AAAD) inhibitor such as carbidopa or benserazide. These agents increase the strength and duration of oxitriptan. An investigational combination formulation is oxitriptan/carbidopa.
Research
[edit]Psychedelic effects
[edit]5-HTP robustly produces the head-twitch response (HTR) in rodents when administered at relatively high doses.[8][9][10][11] Similarly, intracerebroventricular injection of serotonin, but not peripheral administration of serotonin, produces the HTR.[9][8][11] The HTR is induced by serotonergic psychedelics like lysergic acid diethylamide (LSD) and psilocybin and is a behavioral proxy of psychedelic effects.[12][8]
The HTR of 5-HTP is blocked by serotonin 5-HT2A receptor antagonists, which block the hallucinogenic effects of serotonergic psychedelics in humans, is prevented by aromatic L-amino acid decarboxylase (AAAD) inhibitors, which block conversion of 5-HTP into serotonin, and is potentiated by monoamine oxidase A (MAO-A) inhibitors, which prevent the degradation of serotonin and other endogenous tryptamines.[9][8][10][11] In addition, the HTR of 5-HTP is abolished by indolethylamine N-methyltransferase (INMT) inhibitors, which block conversion of serotonin and other endogenous tryptamines into N-methylated tryptamines, such as N-methylserotonin (NMS; norbufotenin), bufotenin (5-hydroxy-N,N-dimethyltryptamine; 5-HO-DMT), and N,N-dimethyltryptamine (DMT).[8][13][11] These N-methylated tryptamines are well-known for their psychedelic effects, whereas serotonin itself, without biotransformation, does not seem to produce psychedelic effects.[8][11] 5-HTP has not been found to produce psychedelic effects in humans, which has been attributed to the high doses required to produce such effects.[8][10] The 5-HTP doses that produce the HTR in rodents are orders of magnitude higher than the doses of 5-HTP that have been used safely and therapeutically in humans.[10]
The lack of the HTR and psychedelic effects with serotonin itself has been attributed to the fact that these effects appear to be dependent on activation of a population of intracellular 5-HT2A receptors expressed in cortical neurons in the medial prefrontal cortex (mPFC) that lack the serotonin transporter (SERT) and are inaccessible to serotonin.[14][15] Serotonin itself is too hydrophilic to enter serotonergic neurons without the SERT, whereas serotonergic psychedelics and serotonin's N-methylated metabolites and analogues are lipophilic and readily enter these neurons.[14][15] These findings may also explain why selective serotonin reuptake inhibitors (SSRIs) and related serotonergic agents do not produce psychedelic effects.[14]
See also
[edit]References
[edit]- ^ Turner EH, Loftis JM, Blackwell AD (March 2006). "Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan". Pharmacology & Therapeutics. 109 (3): 325–38. doi:10.1016/j.pharmthera.2005.06.004. PMID 16023217. S2CID 2563606.
- ^ Rahman MK, Nagatsu T, Sakurai T, Hori S, Abe M, Matsuda M (October 1982). "Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in rats". Japanese Journal of Pharmacology. 32 (5): 803–11. doi:10.1254/jjp.32.803. PMID 6983619.
- ^ Bouchard S, Bousquet C, Roberge AG (September 1981). "Characteristics of dihydroxyphenylalanine/5-hydroxytryptophan decarboxylase activity in brain and liver of cat". Journal of Neurochemistry. 37 (3): 781–7. doi:10.1111/j.1471-4159.1982.tb12555.x. PMID 6974228. S2CID 43853143.
- ^ Nakatani Y, Sato-Suzuki I, Tsujino N, Nakasato A, Seki Y, Fumoto M, Arita H (May 2008). "Augmented brain 5-HT crosses the blood-brain barrier through the 5-HT transporter in rat". The European Journal of Neuroscience. 27 (9): 2466–72. doi:10.1111/j.1460-9568.2008.06201.x. PMID 18445233. S2CID 18940166.
- ^ Bouchard S, Roberge AG (July 1979). "Biochemical properties and kinetic parameters of dihydroxyphenylalanine--5-hydroxytryptophan decarboxylase in brain, liver, and adrenals of cat". Canadian Journal of Biochemistry. 57 (7): 1014–8. doi:10.1139/o79-126. PMID 39668.
- ^ Amamoto T, Sarai K (September 1976). "On the tryptophan-serotonin metabolism in manic-depressive disorders. Changes in plasma 5-HT and 5-HIAA levels and urinary 5-HIAA excretion following oral loading of L-5HTP in patients with depression". Hiroshima Journal of Medical Sciences. 25 (2–3): 135–40. PMID 1088369.
- ^ "5-Hydroxytryptophan". University of Maryland Medical Center. Archived from the original on 6 January 2010. Retrieved 21 January 2010.
- ^ a b c d e f g Kozlenkov, Alexey; González-Maeso, Javier (2013). "Animal Models and Hallucinogenic Drugs". The Neuroscience of Hallucinations. New York, NY: Springer New York. p. 253–277. doi:10.1007/978-1-4614-4121-2_14. ISBN 978-1-4614-4120-5.
- ^ a b c Schmid, Cullen L.; Bohn, Laura M. (2018). "βArrestins: Ligand-Directed Regulators of 5-HT2A Receptor Trafficking and Signaling Events". 5-HT2A Receptors in the Central Nervous System. Cham: Springer International Publishing. p. 31–55. doi:10.1007/978-3-319-70474-6_2. ISBN 978-3-319-70472-2.
- ^ a b c d Jaster AM, de la Fuente Revenga M, González-Maeso J (July 2022). "Molecular targets of psychedelic-induced plasticity". J Neurochem. 162 (1): 80–88. doi:10.1111/jnc.15536. PMC 9068831. PMID 34741320.
- ^ a b c d e Schmid CL, Bohn LM (October 2010). "Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo". J Neurosci. 30 (40): 13513–24. doi:10.1523/JNEUROSCI.1665-10.2010. PMC 3001293. PMID 20926677.
- ^ Canal CE, Morgan D (2012). "Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: a comprehensive history, a re-evaluation of mechanisms, and its utility as a model". Drug Test Anal. 4 (7–8): 556–576. doi:10.1002/dta.1333. PMC 3722587. PMID 22517680.
- ^ Halberstadt AL, Geyer MA (2018). "Effect of Hallucinogens on Unconditioned Behavior". Curr Top Behav Neurosci. 36: 159–199. doi:10.1007/7854_2016_466. PMC 5787039. PMID 28224459.
- ^ a b c Sapienza, Jacopo (13 October 2023). "The Key Role of Intracellular 5-HT2A Receptors: A Turning Point in Psychedelic Research?". Psychoactives. 2 (4): 287–293. doi:10.3390/psychoactives2040018. ISSN 2813-1851.
- ^ a b Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE (February 2023). "Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors". Science. 379 (6633): 700–706. doi:10.1126/science.adf0435. PMC 10108900. PMID 36795823.
