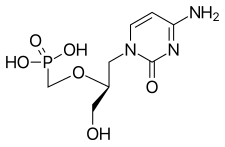
Cidofovir
 | |
| Clinical data | |
|---|---|
| Trade names | Vistide |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | complete |
| Protein binding | <6% |
| Elimination half-life | 2.6 hours (active metabolites: 15–65 hours) |
| Excretion | renal The above pharmacokinetic parameters are measured for cidofovir used in conjunction with probenecid.[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.433 |
| Chemical and physical data | |
| Formula | C8H14N3O6P |
| Molar mass | 279.189 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | -97.3 |
| Melting point | 260 °C (500 °F) |
| |
| |
| (verify) | |
Cidofovir, brand name Vistide, is a topical or injectable antiviral medication primarily used as a treatment for cytomegalovirus (CMV) retinitis (an infection of the retina of the eye) in people with AIDS.[4][5]
Cidofovir was approved for medical use in 1996.[6]
Medical use
[edit]DNA virus
[edit]Its only indication that has received regulatory approval worldwide is cytomegalovirus retinitis.[4][5] Cidofovir has also shown efficacy in the treatment of aciclovir-resistant HSV infections.[7] Cidofovir has also been investigated as a treatment for progressive multifocal leukoencephalopathy with successful case reports of its use.[8] Despite this, the drug failed to demonstrate any efficacy in controlled studies.[9] Cidofovir might have anti-smallpox efficacy and might be used on a limited basis in the event of a bioterror incident involving smallpox cases.[10] Brincidofovir, a cidofovir derivative with much higher activity against smallpox that can be taken orally has been developed.[11] It has inhibitory effects on varicella-zoster virus replication in vitro although no clinical trials have been done to date, likely due to the abundance of safer alternatives such as aciclovir.[12] Cidofovir shows anti-BK virus activity in a subgroup of transplant recipients.[13] Cidofovir is being investigated as a complementary intralesional therapy against papillomatosis caused by HPV.[14][15]
It first received FDA approval on 26 June 1996,[16] TGA approval on 30 April 1998[5] and EMA approval on 23 April 1997.[17]
It has been used topically to treat warts.[18]
Other
[edit]It has been suggested as an antitumour agent, due to its suppression of FGF2.[19][20][21]
Administration
[edit]Cidofovir is only available as an intravenous formulation. Cidofovir is to be administered with probenecid which decreases side effects to the kidney.[22] Probenecid mitigates nephrotoxicity by inhibiting organic anion transport of the proximal tubule epithelial cells of the kidney.[23] In addition, hydration must be administered to patients receiving cidofovir. 1 liter of normal saline is recommended in conjunction with each dose of cidofovir.[22]
Side effects
[edit]The major dose-limiting side effect of cidofovir is nephrotoxicity (i.e., kidney damage).[24] Other common side effects (occurring in >1% of people treated with the drug) include:[4][24]
- Nausea
- Vomiting
- Neutropenia
- Hair loss
- Weakness
- Headache
- Chills
- Decreased intraocular pressure
- Uveitis
- Iritis
Whereas uncommon side effects include: anaemia and elevated liver enzymes and rare side effects include: tachycardia and Fanconi syndrome.[24] Probenecid (a uricosuric drug) and intravenous saline should always be administered with each cidofovir infusion to prevent this nephrotoxicity.[25]
Contraindications
[edit]Hypersensitivity to cidofovir or probenecid (as probenecid needs to be given concurrently to avoid nephrotoxicity).[4]
Interactions
[edit]It is known to interact with nephrotoxic agents (e.g. amphotericin B, foscarnet, IV aminoglycosides, IV pentamide, vancomycin, tacrolimus, non-steroid anti-inflammatory drugs, etc.) to increase their nephrotoxic potential.[4][5] As it must be given concurrently with probenecid it is advised that drugs that are known to interact with probenecid (e.g. drugs that probenecid interferes with the renal tubular secretion of, such as paracetamol, aciclovir, aminosalicylic acid, etc.) are also withheld.[5]
Mechanism of action
[edit]Its active metabolite, cidofovir diphosphate, inhibits viral replication by selectively inhibiting viral DNA polymerases.[5] It also inhibits human polymerases, but this action is 8–600 times weaker than its actions on viral DNA polymerases.[5] It also incorporates itself into viral DNA, hence inhibiting viral DNA synthesis during reproduction.[5]
It possesses in vitro activity against the following viruses:[26]
- Human herpesviruses
- Adenoviruses
- Human poxviruses (including the smallpox virus)
- Human papillomavirus
History
[edit]Cidofovir was discovered at the Institute of Organic Chemistry and Biochemistry, Prague, by Antonín Holý, and developed by Gilead Sciences[27] and is marketed with the brand name Vistide by Gilead in the US, and by Pfizer elsewhere.
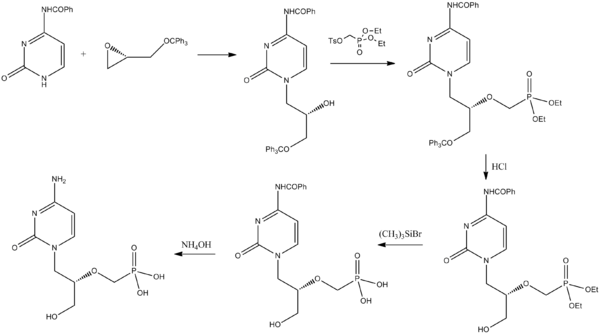
Synthesis
[edit]Cidofovir can be synthesized from a pyrimidone derivative and a protected derivative of glycidol.[28]
See also
[edit]- Brincidofovir, a prodrug of cidofovir
References
[edit]- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Drug and medical device highlights 2018: Helping you maintain and improve your health". Health Canada. 14 October 2020. Retrieved 17 April 2024.
- ^ Cundy, Kenneth C. "Clinical Pharmacokinetics of the Antiviral Nucleotide Analogues Cidofovir and Adefovir." Clinical Pharmacokinetics 36.2 (1999): 127–143.
- ^ a b c d e "Vistide (cidofovir) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 4 February 2014.
- ^ a b c d e f g h "Product Information VISTIDE®". TGA eBusiness Services. Gilead Sciences Pty Ltd. 3 September 2013. Retrieved 5 February 2014.
- ^ Long SS, Prober CG, Fischer M (2012). Principles and Practice of Pediatric Infectious Disease. Elsevier Health Sciences. p. 1502. ISBN 978-1437727029.
- ^ Chilukuri S, Rosen T (April 2003). "Management of acyclovir-resistant herpes simplex virus". Dermatologic Clinics. 21 (2): 311–320. doi:10.1016/S0733-8635(02)00093-1. PMID 12757254.
- ^ Segarra-Newnham M, Vodolo KM (June 2001). "Use of cidofovir in progressive multifocal leukoencephalopathy". The Annals of Pharmacotherapy. 35 (6): 741–744. doi:10.1345/aph.10338. PMID 11408993. S2CID 32026770.[permanent dead link]
- ^ De Gascun CF, Carr MJ (2013). "Human polyomavirus reactivation: disease pathogenesis and treatment approaches". Clinical & Developmental Immunology. 2013: 373579. doi:10.1155/2013/373579. PMC 3659475. PMID 23737811.
- ^ De Clercq E (July 2002). "Cidofovir in the treatment of poxvirus infections". Antiviral Research. 55 (1): 1–13. doi:10.1016/S0166-3542(02)00008-6. PMC 9533828. PMID 12076747.
- ^ Bradbury J (March 2002). "Orally available cidofovir derivative active against smallpox". Lancet. 359 (9311): 1041. doi:10.1016/S0140-6736(02)08115-1. PMID 11937193. S2CID 22903225.
- ^ Magee WC, Hostetler KY, Evans DH (August 2005). "Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate". Antimicrobial Agents and Chemotherapy. 49 (8): 3153–3162. doi:10.1128/AAC.49.8.3153-3162.2005. PMC 1196213. PMID 16048917.
- ^ Araya CE, Lew JF, Fennell RS, Neiberger RE, Dharnidharka VR (February 2006). "Intermediate-dose cidofovir without probenecid in the treatment of BK virus allograft nephropathy". Pediatric Transplantation. 10 (1): 32–37. doi:10.1111/j.1399-3046.2005.00391.x. PMID 16499584. S2CID 24131709.
- ^ Broekema FI, Dikkers FG (August 2008). "Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis". European Archives of Oto-Rhino-Laryngology. 265 (8): 871–879. doi:10.1007/s00405-008-0658-0. PMC 2441494. PMID 18458927.
- ^ Soma MA, Albert DM (February 2008). "Cidofovir: to use or not to use?". Current Opinion in Otolaryngology & Head and Neck Surgery. 16 (1): 86–90. doi:10.1097/MOO.0b013e3282f43408. PMID 18197029. S2CID 22895067.
- ^ "Cidofovir Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 5 February 2014.
- ^ "Vistide : EPAR – Product Information" (PDF). European Medicines Agency. Gilead Sciences International Ltd. 7 November 2013. Archived from the original (PDF) on 22 February 2014. Retrieved 5 February 2014.
- ^ Fernández-Morano T, del Boz J, González-Carrascosa M, Tortajada B, de Troya M (December 2011). "Topical cidofovir for viral warts in children". Journal of the European Academy of Dermatology and Venereology. 25 (12): 1487–1489. doi:10.1111/j.1468-3083.2010.03961.x. PMID 21261749. S2CID 32295082.
- ^ Kern ER (January 2003). "In vitro activity of potential anti-poxvirus agents". Antiviral Research. 57 (1–2): 35–40. doi:10.1016/s0166-3542(02)00198-5. PMC 9628899. PMID 12615301. S2CID 23136929.
- ^ Andrei G, Snoeck R (December 2010). "Cidofovir Activity against Poxvirus Infections". Viruses. 2 (12): 2803–2830. doi:10.3390/v2122803. PMC 3185586. PMID 21994641.
- ^ "Interim Clinical Guidance for the Treatment of Monkeypox". U.S. Centers for Disease Control and Prevention. 26 May 2022.
- ^ a b "Details" (PDF). www.gilead.com. Retrieved 2019-06-05.
- ^ Lacy SA, Hitchcock MJ, Lee WA, Tellier P, Cundy KC (August 1998). "Effect of oral probenecid coadministration on the chronic toxicity and pharmacokinetics of intravenous cidofovir in cynomolgus monkeys". Toxicological Sciences. 44 (2): 97–106. doi:10.1006/toxs.1998.2481. PMID 9742650.
- ^ a b c Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ "Vistide (cidofovir)" (PDF) (package insert). Gilead Sciences. September 2010. Dosage and Administration: Dosage.
- ^ Safrin S, Cherrington J, Jaffe HS (September 1997). "Clinical uses of cidofovir". Reviews in Medical Virology. 7 (3): 145–156. doi:10.1002/(SICI)1099-1654(199709)7:3<145::AID-RMV196>3.0.CO;2-0. PMID 10398479. S2CID 32366514.
- ^ "Press Releases: Gilead". Archived from the original on 2013-02-08. Retrieved 2007-12-05.
- ^ Brodfuehrer PR, Howell HG, Sapino Jr C, Vemishetti P (1994). "A practical synthesis of (S)-HPMPC". Tetrahedron Letters. 35 (20): 3243. doi:10.1016/S0040-4039(00)76875-4.